Your selection
Research, Patient care / 26.07.2023
New muscle therapy gets fast-track boost
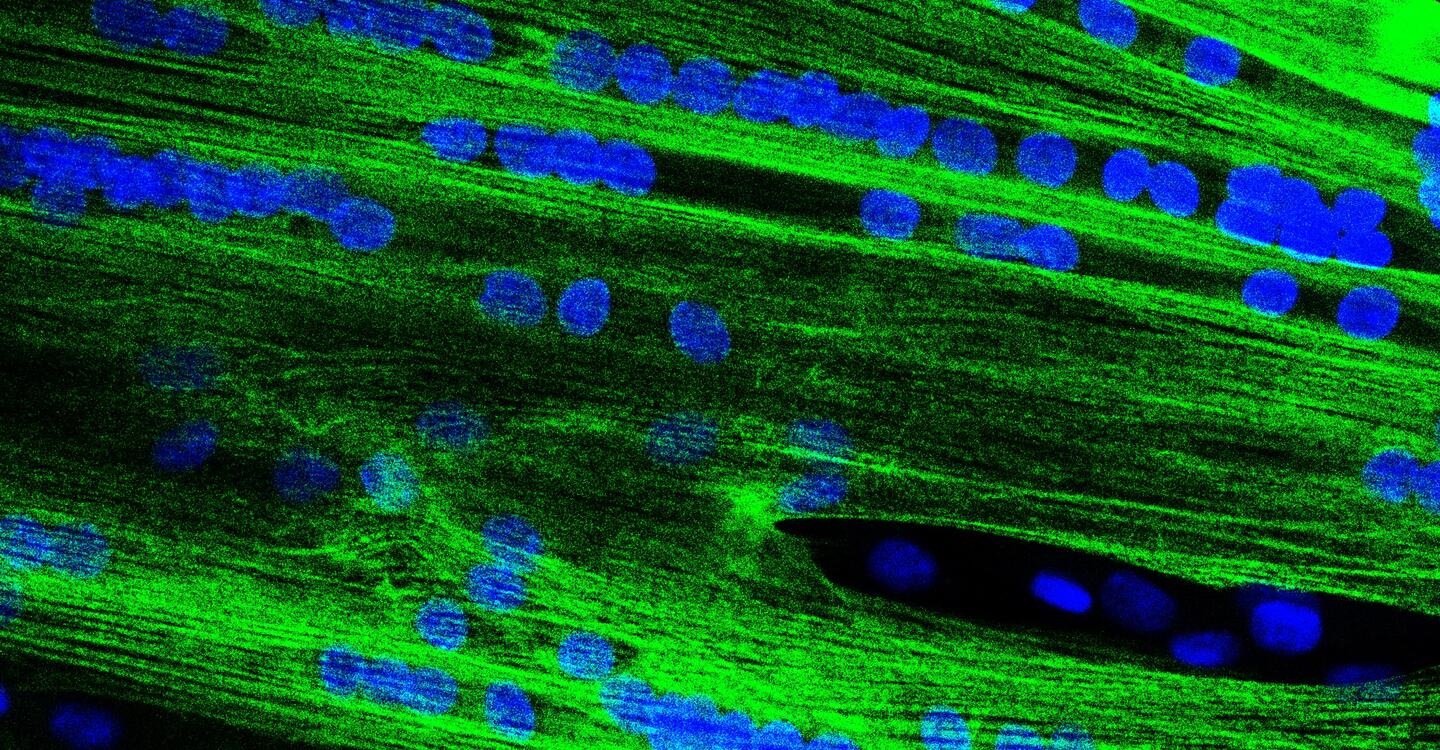
The stem cell therapy developed by MyoPax, a spin-off from the Max Delbrück Center and Charité, could soon be helping children with previously incurable muscle diseases thanks to an accelerated approval process. The necessary clinical trial will start in the fall.
To help bring therapies for rare muscle diseases in children to market sooner, the Berlin-based start-up MyoPax, a spin-off from the Max Delbrück Center and Charité – Universitätsmedizin Berlin, has now received a boost from the U.S. Food and Drug Administration (FDA). The company has been granted the FDA’s orphan drug designation (ODD) and rare pediatric disease designation (RPDD), both of which offer multiple regulatory and financial advantages – including fast-track approval status and, eventually, market exclusivity.
But first, the researchers must test their new therapeutic approach in a clinical trial sponsored by Charité. The German Federal Ministry of Education and Research and ForTra gGmbH of the Else Kröner-Fresenius Foundation are funding the trial, which will start with the first patients in the fall and should be completed by 2026.
The result of many years of research
The innovative muscle stem cell technology selected by the FDA is based on years of research by Professor Simone Spuler and her Myology Lab team at the Experimental and Clinical Research Center (ECRC), a joint institution of the Max Delbrück Center and Charité. Spuler founded MyoPax last year, together with Dr. Verena Schöwel-Wolf. Their goal is to develop therapies for local muscle defects, acute muscle wasting and hereditary muscular dystrophies that are currently incurable or for which adequate treatment does not yet exist.
With their stem cell therapy, the team wants to help children who suffer from exstrophy-epispadias complex (EEC). This spectrum of rare congenital disorders is characterized by the usually hollow bladder lying inverted and opened like a plate on the abdominal wall. In addition to malformations of the abdominal muscles, pelvic bones, urethra and external genitalia, the bladder sphincter also remains underdeveloped. This muscle defect is due to delayed cell migration during embryonic development and causes lifelong incontinence. About one in 11,000 children is born with EEC and the malformations are currently surgically corrected. Often, further surgery is required to improve bladder function. With the help of MyoPax’s new stem cell therapy, however, bladder sphincter muscle can be rebuilt, thus allowing the patient to regain long-term bladder control.
“Receiving this recognition from the FDA is an important milestone in our work,” says Spuler. “It confirms that our new therapeutic approach has the potential to improve the lives of young patients with EEC and other muscle diseases – as well as the lives of their parents.”
With her second company, MyoPax Denmark ApS, Spuler has been accepted into the incubator program of the BioInnovation Institute (BII) Foundation in Copenhagen, which provides financial and strategic support to help the company keep up with international competition. Meanwhile, MyoPax is preparing for expansion to the United States.
Text: Jana Ehrhardt-Joswig
Source: Press Release Max Delbrück Center
New muscle therapy gets fast-track boost
Overview News
News Buch Berlin
The Imaging Innovation Center hits a milestone
The Max Delbrück Center, Berlin, celebrates its new, innovative research building with a “topping-out ceremony.” The Imaging Innovation Center, designed by the architectural firm heinlewischer, will h...
more ...Giant with a Ceramic Heart
A new NMR spectrometer has been in operation at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) since the end of May.
more ...Holger Gerhardt new member of the DZHK Board of Directors
Professor Holger Gerhardt from the DZHK partner site Berlin was newly elected to the Board of Directors by the General Assembly: He will take office on 1 July 2024.
more ...Events Buch Berlin
17.08.2024, 21:15
Open Air Sommerkino in Hobrechtsfelde: "Dirty Dancing"
Kultfilm von 1987
more ...10.09.2024, 09:00
From Target to Market - The GLA Biotech & Pharma Summer School
The 4-day intensive course provides a comprehensive overview of the entire drug development process in biotechnology and in the pharmaceutical industry – from the idea to the market.
more ...12.09.2024, 09:00
Realtime PCR und digital PCR Kurs
Der RealTime PCR und Digital PCR Kurs richtet sich an erfahrene PCR Anwender*innen und an Einsteiger*innen. Wichtige PCR Grundlagen werden erörtert, bevor die RealTime PCR besprochen und Genexpression...
more ...







